
前期介绍了硫醇的制备方法(点击查看),目前有两种常用的方法将脂肪硫醇转变为脂肪磺酰氯,一是用NaClO4在酸性溶液中处理得来。另一种是在酸性介质中通入氯气制得。

Asolution of 4-(7-Bromo-2-ethoxymethyl-imidazo[4,5-c]quinolin-1-yl)-butane-1-thiol(1.73 g, 4.39 mmol) inconcentrated hydrochloric acid (7.5 ML) and water (5 mL) was cooled to 0 oC.A solution of sodium chlorate (0.61 g, 5.7 mmol) in water (2. 5 mL) was added dropwise withvigourous stirring over a period of three minutes. The reaction was stirred at0 oC for 90 minutes then diluted with dichloromethane (50 mL).Aqueous potassium carbonate (8 mL of 6M)was slowly added to adjust the mixture to pH equal 5. Dichloromethane (100 mL)and water (75 mL) were added, and the reaction was allowed to warm to ambienttemperature with stirring. The aqueous layer was separated and extracted withdichloromethane (3 x 40 mL). The combined organic fractions were dried overmagnesium sulfate, filtered, and concentrated under reduced pressure to provide1.61 g of4-(7-Bromo-2-ethoxymethyl-imidazo[4,5-c]quinolin-1-yl)-butane-1-sulfonylchloride as a tan solid.
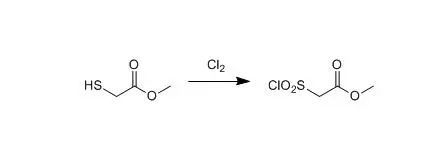
To a solution of methylthioglycolate (1.3kg, 12.25mol) in dichloromethane (9litres) was added ice (4.5 litres). Chlorine gas was bubbled gently through thesolution, maintaining the temperature below 5.deg.C until the solutionmaintained a slight green colouration. The solution was degassed with nitrogento remove excess chlorine, the organic phase collected and the solvent removedunder reduced pressure to give the subtitle compound (1.758 kg, 83 percent) which was usedwithout further purification.
本文非原创内容,版权归原作者所有,如涉及版权问题,请联系本号删除。
