
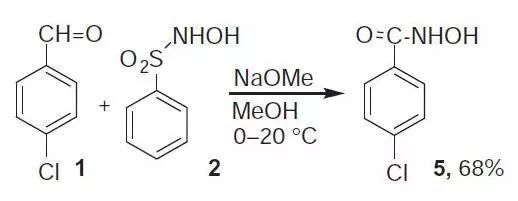
碱性条件下,醛和N-磺酰基羟胺反应生成羟肟酸的反应。反应的副产物是亚磺酸。 此反应由意大利化学家Angelo Angeli 和 Enrico Rimini在1896年发现。此反应可以用于醛的颜色测试。
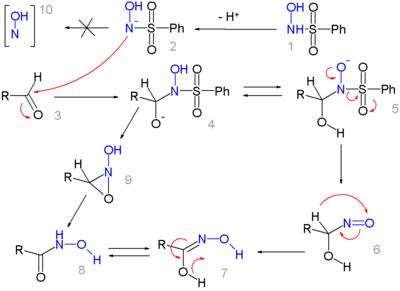
可能通过两种路径反应。

p-Chlorobenzene hydroxamic acid (5).6 To an ice-cold solution of N-hydroxybenzene sulfonamide 2 (730 mg, 4.2 mmol) in MeOH was added dropwise NaOMe-MeOH solution (4.36 mL, 8.4 mmol, 1.93 M). p-Chlorobenzaldehyde 1 (562 mg, 4 mmol) in MeOH (4 mL) was then added and the reaction mixture was warmed to r.t. MeOH was removed in vacuo, the residue was dissolved in ether (200 mL) and the organic layer was extracted with 2M NaOH. The aq layer was acidified with conc HCl and extracted with EA. The solution was concentrated to give product 5 (68%).
【Hassner A, J Org Chem, 1970, 35, 1962】
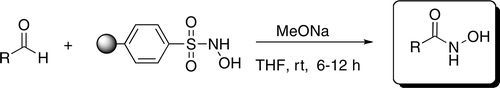
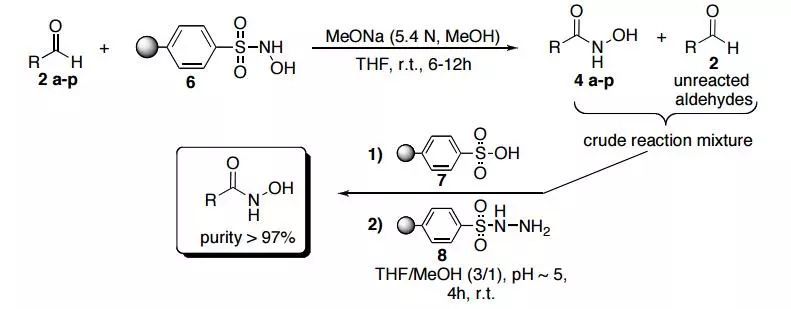
The resin 6 previously obtained (0.6 mmol) was swelled in 5 mLof THF, treated with a 5.4 N solution of NaOMe (1.2 mmol) in MeOH (0.22 mL),shaken at r.t. for 10 min and then reacted with p-chlorobenzaldehyde 2e (42 mg, 0.3mmol). After 6 h, the resin was filtered off and alternatively rinsed with THF (4 x 1mL) and MeOH (3x1 mL). The combined extract (THF:MeOH 3:1) was transferred toanother tube and Dowex® 50WX2-400 ion-exchange resin 7 (150 mg) wasportionwise added to adjust the pH to ~ 5. The ion exchange resin was collected byfiltration, the solution treated with Ps-Ts-NHNH2 8 (300 mg, 0.9 mmol) and thesuspension shaken at room temperature for additional 4 hours. The resin was filteredout and washed with THF (2x), MeOH (2 x), DCM (2 x) and MeOH (2x). The filtratewas concentrated in vacuo, diluted with DCM (2mL) and filtered through a small plug(1.00 cm) of silica gel with Hexane/AcOEt (1:1). Finally, the solution afterevaporation of the solvent, gave the desired N-hydroxyamide 4e (50.3 mg, 98%respect to 2e) as the sole product in. The product gives a red colour with FeCl3 too.
【Porecheddu A, J Org Chem, 2006, 71, 7057】
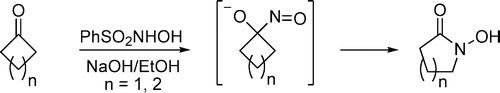
A solution of degassed sodium hydroxide (2N, 80mmol, 40 mL) was added to a solution of cyclobutanone (1 g, 14.27 mmol) in EtOH (20mL) at 0°C. To this mixture a solution of Piloty’s acid (4.94 g, 28.53 mmol) in EtOH (20mL) was added drop wise over 30 min. The reaction mixture was stirred at 0°C for 4 hand at room temperature for another 18 h. The ethanol was removed under vacuum andthe water layer was extracted with diethyl ether to remove the unreacted cyclobutanone.The aqueous layer was acidified to pH 5.5 with 2N HCl and extracted with CHCl3 (6 x 50mL). The CHCl3 layer was dried with MgSO4 and was concentrated to a solid cruderesidue that was purified by flash chromatography to give the desired hydroxamic acid 3as a pale white solid product (0.565 g, 39%)
【King SB, Org Lett, 2009, 11, 4580】
编译自:Organic Syntheses Based On Name Reactions, 3RdEd, A. Hassner, Page 10.
