
Anelli在1987年报道了在二氯甲烷-水两相溶剂中利用TEMPO( ca. 1% mol), NaOCl,KBr 和NaHCO3将醇氧化为醛酮的反应。
TEMPO = 四甲基哌啶氧化物。2,2,6,6-四甲基哌啶氧自由基是一种市售的很稳定的氮氧自由基化合物,在此反应中充当催化剂。
反应中加入碳酸氢钠的作用是保持体系中的pH 在 8.6–9.5 ,因为市售的次氯酸钠的pH=12.7,碱性太强影响反应进行,当反应底物对碱敏感时可以用0.1 M HCl调节pH到6.5-7.5。
反应中加入KBr的作用可能是会产生一些HOBr,加速反应。反应一般在0℃到室温反应,反应速度很快(氧化到醛一般3min,氧化为酮7-10min)。
仲醇氧化到酮就会停止,但醛可能会继续氧化生成羧酸,但反应速度很慢,另外必须加入过量的次氯酸钠才可以继续氧化。具体内容参考往期发布的:TEMPO氧化。
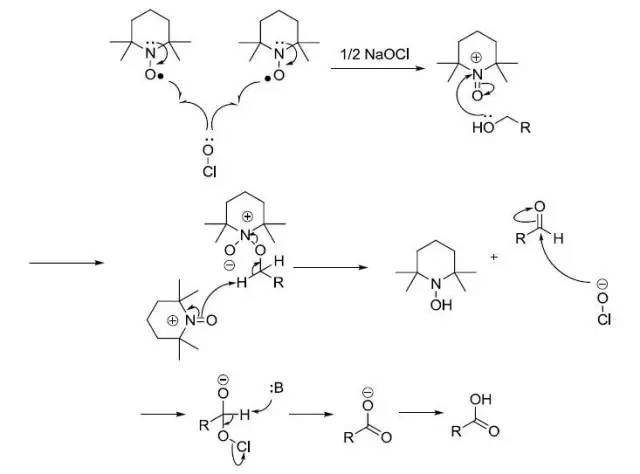

对于一些位阻较大的醇,反应中加入季铵盐作为相转移催化剂,可以加速反应。但是也会加快过氧化过程生成羧酸。
次氯酸钠会产生HOCl,可以和烯烃等一些富电子的底物反应。此过程可以通过TEMPO-PhI(OAc)2(Protocol of Piancatelli and Margarita)法解决。
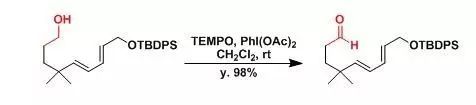
【Jauch, J. Angew. Chem., Int. Ed.2000, 39, 2764.】
操作步骤
General Procedure for Oxidation of Alcohols with TEMPO-NaOCl (Anelli’s Protocol)
A two phase system consisting of a ca. 0.2–2.9 M solution of 1 equiv. of the alcohol in DCM, containing ca. 0.2–5% mol, typically 1–2% mol—of TEMPO and a ca. 0.02–2.6 M solution of ca. 0.02–0.5 equiv., typically 0.1 equiv. of KBr or NaBr in water, is vigorously stirred over a water ice bath (0C) or an ice-salt bath (-10C).
Over this two phase system, ca. 1.09–1.4 equiv. of NaOCl in a fresh solution, prepared by adjusting a ca. 5–13% aqueous solution of NaOCl to a pH of 8.6–9.5 by addition of an aqueous solution of NaHCO3, are slowly added. When starting compound is consumed, the organic phase is separated and the aqueous phase is washed with DCM. The collected organic phases are washed with a Na2S2O3aqueous solution and water or brine.
后处理
Optionally, the collected organic phases may be washed with a solution of ca. 0.2–2.5 equivalents of KI in 10–20% hydrochloric acid, before washing with the sodium thiosulfate solution. Finally, the organic solution is dried (Na2SO4or MgSO4) and concentrated, giving a residue that may need further purification.
ØGeneral Procedure for Oxidation of Alcohols TEMPO-PhI(OAc)2(Protocol of Piancatelli and Margarita)
A ca. 0.04–1 M solution of the alcohol in DCM, containing 0.09–0.2 equiv., typically 0.1 equiv. of TEMPO and 1.1–5 equiv., typically 1.1 equiv. of PhI(OAc)2(BAIB), is stirred at room temperature till most of the starting alcohol is consumed. Then, some DCM may be optionally added in order to facilitate subsequent washings. The reaction mixture is washed with an aqueous Na2S2O3 solution. Optionally, the organic phase can be washed with aqueous NaHCO3and brine. Finally, the organic solution is dried (Na2SO4) and concentrated, giving a residue that may need further purification.
此条件下反应不要求无水,反应时间2-12h。
反应机理
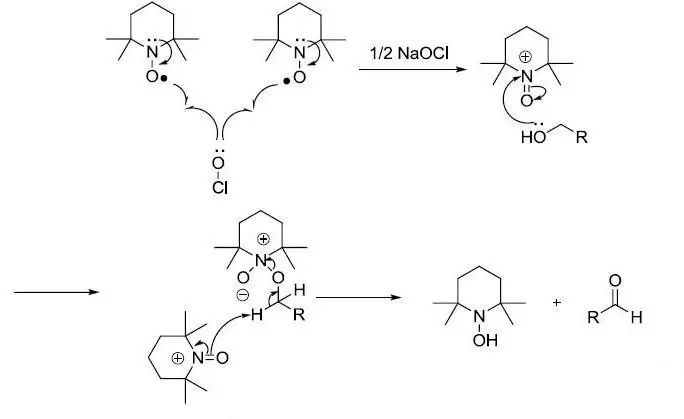
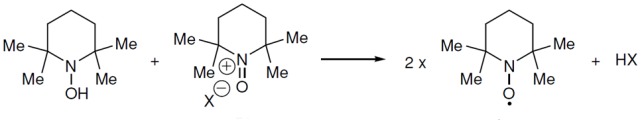
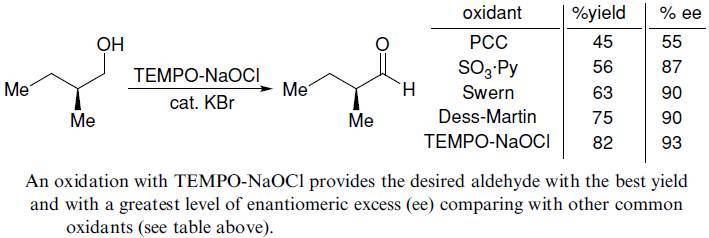

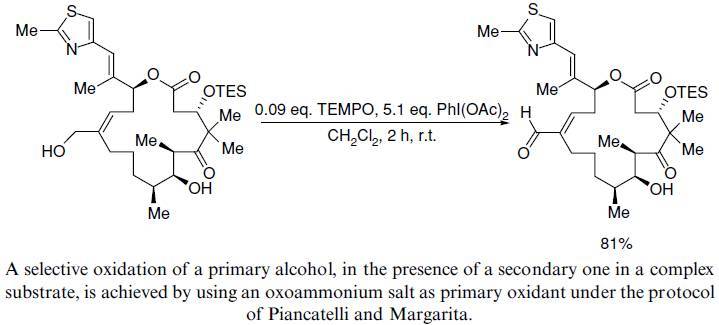
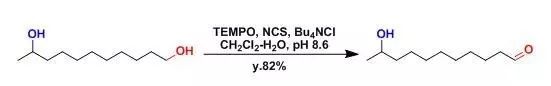
【J. Org. Chem.1996, 61, 7452】
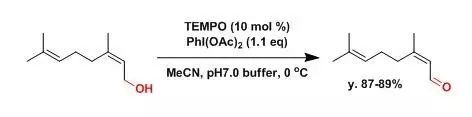
【Org. Synth.2006, 83, 18】
参考文献
一、common oxidation reagents, Yue Xu, Sundia Meditech
二、化学空间:TEMPO的氧化反应
