
通过Mitsunobu反应取代羟基合成叠氮化物,然后还原,便能得到伯胺。

【Afonso, C. A; Barros, M. T; Maycock, C. D. Tetrahedron.Lett. 1999, 40, 801.】
由于叠氮酸使用不方便,一个替代方法是用diarylphosphoryl azide(DPPA)作为叠氮基团的来源。Taber 和Decher 通过这个方法得到了相应的叠氮化合物,产率还不错。
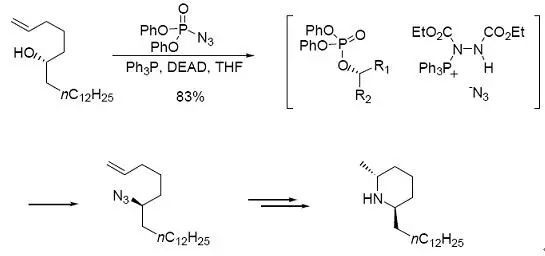
【Taber, D. F; Decker,P. B. J. Org. Chem. 1988, 53, 2968.】
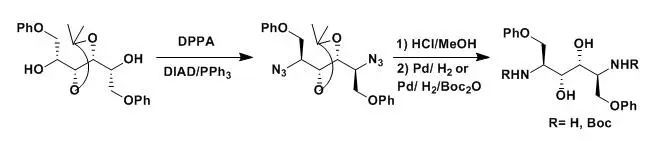
To a cooled solution (-5oC)of DIAD (7.9 g, 93 mmol) in THF (5 mL) was added the substituted alchol (7.06g, 18.7 mmol) and PPh3(10.3 g, 39.1 mmol). After 15 min, diphenylphosphorazidate (DPPA, 12.86 g, 46.77mmol) was added and the reaction mixturewas allowed to warm to room temperature. After stirring overnight, the solventwas removed in vacuo to give a yellow oil. The crude material was purified byflash column chromatograghy (2:1,PE/Tol) to give the desired product (7.28 g,91%) as a colorless oil.
The de-protection andhydrogenation were routine operations.
【Zuccarello, G; Bouzide, A; J. Org. Chem. 1998, 63, 4898.】
本文非原创内容,版权归原作者所有。
Mitsunobu醚化反应
