
卤代烃类物质性质概述
物质性质
1. CH3Cl, CH3Br, C2H5Cl 在常温常压下是气体. 其他的卤代烃多为液体, 且物质的质量越大, 其沸点越高. are all gases at room temperature and pressure. The other haloalkanes are liquids with boiling points related to molar mass.
2. 卤代烃不溶于水. They are all immiscible with water.
3. 卤代烃类物质通式为CnH2n+1X (where X = halogen atom)
4. 由于卤素较强的电负性, 因此卤代烃多为极性分子. Haloalkanes are polar due to the inductive effect of the halogen atom. Since the halogens are more electronegative than carbon, they have a greater share of the electrons in the C-X bond.
示意图如下:

(1) 根据卤素在卤代烃中的位置不同, 有三种主要的卤代烃:
There are three distinct molecular environments for the halogen atom:
伯卤代烃:
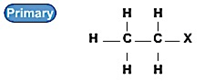
仲卤代烃:
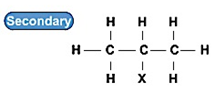
叔卤代烃:
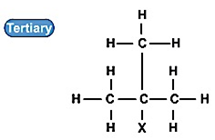
(2) 极性趋势: 叔位的卤代烃>仲卤代烃>伯位的卤代烃
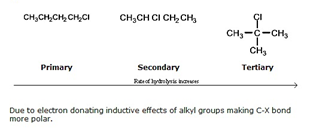
例如: Which of the following haloalkanes will have the greatest polarity?
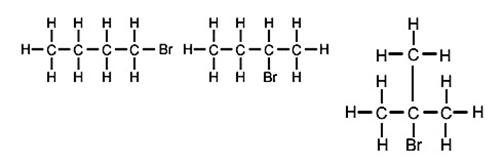
The third example (tertiary halogenoalkane) has the greatest polarity. This is because the positive carbon ion (carbocation) is stabilised by the inductive effect of the three other bound carbons.
机理分析: 卤素的电负性偏大, 因此卤代烃的比同根的烃类物质更活泼. The polarity of the C-X bond results in haloalkanes being much more reactive than their parent alkanes. Therefore they are of greater importance industrially.
◆◆ ◆
化学性质Chemical properties
1. C-X 键上可发生的反应Types of reaction occurring at C-X bond:
a. Nucleophilic substitution reactions亲核取代
b. Elimination reactions消去反应
(1) Nucleophilic substitution
卤素的电负性比碳元素大,吸引电子能力更强, 导致碳元素偏正电. 因此,碳原子会被亲核试剂攻击, 使得反应发生. The inductive effect of the halogen atom results in a positive charge on the carbon atom to which it is attached. Hence, this carbon atom is readily attacked by nucleophiles.
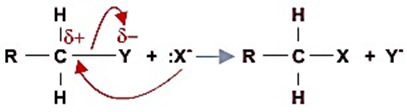
亲核反应举例:
a) 被 亲核试剂OH-亲核取代→水解反应
Attack by OH- (or water): Hydrolysis
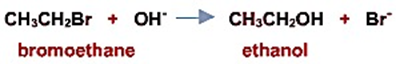
卤代烃在碱性溶液中易于发生水解反应, 纯水中反应非常缓慢. 在使用氢氧化钠/氢氧化钾水溶液回流条件下, 速度变慢,但是产率更高. The haloalkanes are attacked only slowly by water. The rate is much faster, but a poor yield is obtained if the haloalkane is refluxed with aqueous sodium or potassium hydroxide.

b) 被亲核试剂氰基攻击:Formation of nitriles: attack by CN-
使用氰化钾的乙醇溶液回流,可产生If a haloalkanes is refluxed with an alcoholic solution of KCN, an acid nitrile is formed: R-C=N
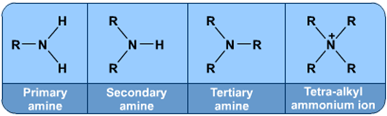
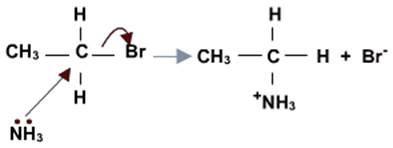
c) 被亲核试剂氨攻击,产物为胺类物质. Attack by ammonia NH3: Products are amines.
使用加热条件下的氨乙醇溶液回流, 会产生胺和铵盐混合物. If a haloalkane is heated with an alcoholic solution of ammonia in a sealed tube, a mixture of products is formed. The mixture consists of amines and amine salts.
伯胺的产生机制:
Mechanism for production of primary amines.
Nucleophilic attack by ammonia:
Abstraction of a proton (taken by a base):
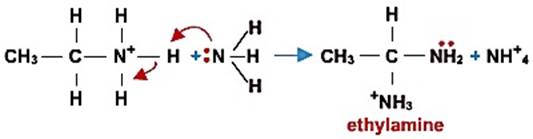
(2)消去反应Elimination reactions
碱性条件下回流可产生醇类物质.When haloalkanes are heated with aqueous solution of potassium or sodium hydroxide, the major product is the alcohol, produced by nucleophilic displacement of the halogen by OH-.
加热条件下, 使用浓缩的氢氧化钠醇溶液回流, 可在 C-X 键的位置发生消去反应, 脱去 HX 小分子, 产生 C=C 双键. If the reaction conditions are changed so that the haloalkane is heated with concentrated alcoholic potassium hydroxide, the major product is an alkene due to the elimination of hydrogen halide.
以上是我们需要了解的
关于卤代烃类物质性质的基本知识
大家要记牢哦~


毕业几年,
不同工作载浮载沉,
投身于培训业,
跟少年做朋友,
与青年当伙伴。
左手语言培训,
右手理科教学。
青春与未来相伴,
未来投射于新一代,
岂不快哉!
—— 刘欣梦
