
甲酰胺制备醛
有机金属化合物(有机镁、有机锂化合物)可与过量的原甲酸脂反应,首先生成缩醛,继而用硫酸水解成醛,广泛用于脂醛及芳醛的合成,产率达55%-90%。
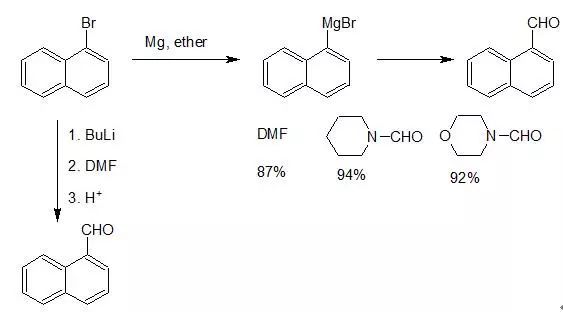
原甲酸脂外,甲酰胺、乙氧亚甲基苯胺(ethoxymethyleneaniline,C6H5N=CHOC2H5)等均为常用的甲酰化试剂。其中以甲酰胺的应用最为常见,常称为Bouveault反应。芳卤或乙烯卤化物与丁基锂发生金属化反应,生成芳基锂或乙烯基锂,后者与二甲基甲酰胺反应,高产率地生成相应的醛。醛或酚醚的邻位氢比较活泼,可与丁基锂直接进行金属化反应,继而甲酰化和水解,是邻羟基或邻烷氧基苯甲醛的良好合成方法。
将卤化物变为镁(Grignard试剂)或锂化物等,进行酰化可以合成醛酮。由有机金属化合物合成醛的甲酰化试剂以甲酸酯类、甲酰胺类用得较普遍,以DMF或N-甲酰哌啶使用较为方便。常用的甲酰化试剂有:FCHO,(HCO)2O,HCOOCOCH3,CH(OCH3)3,HCO2C2H5,HCO2Li,PhN=CHOC2H5,Ph-N(CH3)-CHO,DMF,Py-N(CH3)-CHO,N-甲酰基哌啶,N-甲酰基吗啉。
相关文献
G. A. Olah, Synthesis, 1984, 228;
G. A. Olah, Angew. Chem., Int. Ed. Engl., 20,878,1981; Org. Synthesis., 64, 114, 1985;
M. Bogavac, Tetrahedron Lett., 1984, 1843
E. A. Evans, Chem. Ind., 1957, 1596
Bouveault反应
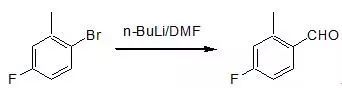
n-BuLi (2.6M in hexane, 57 mL, 143 mmol) was addedover 15 minutes to a THF solution (200 mL) of 2-methyl-4-fluorophenylbromide (24.5 g, 130 mmol), cooled to -78.deg. C. andallowed to stir 1 hour at -78 oC. DMF (26.61 gm, 364 mmol) was then added over 2 minutes, andthe solution was allowed to stir another hour. The reaction was quenched with NH4Cland warmed to room temperature. 10% HCl was added until the solution becameacidic. The mixture was diluted withether and the organic layer washed with water and brine, then dried over MgSO4,filtered and concentrated to give an orange oil. The oil was purified by distillation (bp=69 oC.at 7 mm Hg) to afford aldehyde 4-fluoro-2-methylbenzaldehydeas a clear liquid (13.3 g, 74%). TLC: Rf 0.30 (10% EtOAc in hexane)
weinreb酰胺制备酮
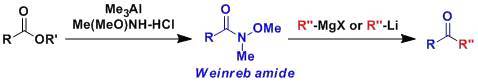
N-甲氧基-N-甲基酰胺俗称Weinreb酰胺、它能与Grignard试剂或有机锂试剂反应生成酮。酰卤或是酯中加入两倍当量的格式试剂或是有机锂试剂的话会得到醇,而Weinreb酰胺则能够避免这种过度的加成。
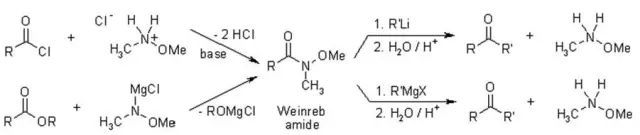
用氢化铝锂或Red-Al、DIBAL等还原Weinreb酰胺的话,会得到醛。
反应机理
Weinreb酰胺和有机金属试剂的加成物由于甲氧基的存在形成一个五元环的螯合物相对稳定,使反应不再继续。

配合物水解则得到酮。

一、由Weinreb酰胺还原合成醛反应
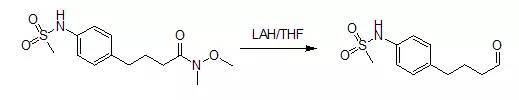
4-[4-(methanesulfonamido)phenyl]butyraldehyde
A mixture of 4.20 g (14 mmol) of4-[4-(methanesulfonamido)phenyl]butyric acid, N-methoxy-N-methylamide and 100 mLof anhydrous tetrahydrofuran was stirred under nitrogen with cooling in an icebath as 17.5 mL (17.5 mmol) of 1Mlithium aluminum hydride in tetrahydrofuran was added gradually by syringe. After 0.75 hours, 70 mL of 5percent potassiumhydrogen sulfate solution (aqueous) was added cautiously by syringe. Themixture was then removed from the ice bath, diluted with 150 ML of water, andshaken with 150 mL of ethyl acetate. The milky aqueous phase was extracted withan additional 50 mL of ethyl acetate. The combined organic fractions werewashed successively with 2*100 mL of 1N hydrochloric acid, then 50 ML ofsaturated aqueous sodium bicarbonate solution, and finally 50 ML of saturatedaqueous sodium chloride solution. The organic phase was dried over magnesiumsulfate, filtered, and concentrated in vacuo. Flash chromatography of theresidue on silica gel (elution with 3:2 hexane-EtOAc) yielded 2.47 g (73%) of an oil; homogeneous by TLC in1:1 hexane-EtOAc).Upon storage in the freezer, solidification occurred (mp 41~44oC.).
【US5756507】
二、由Weinreb酰胺还原合成酮
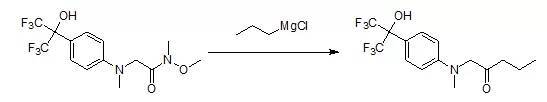
1-(Methyl{4-[2,2,2-trifluoro-1-hydroxy-1-(trifluoromethyl)ethyl]phenyl}amino)-pentan-2-one
To a solution of [N-1--methoxy-N-1-N-2-dimethyl-N-2-- {4- [2,] 2,2- trifluoro-1-hydroxy-1-(trifluoromethyl)ethyl]phenyl}glycinamide (240 mg, 0.64 mmol) in CH2Cl2(3.5 mL) at 0 oC under a nitrogen atmosphere was slowly added asolution of propylmagnesium chloride (1.6 mL, 2M in Et2O). After stirring 3 hours, the reaction mixturewas poured into 1 M HCI andextracted twice with EtOAc. The organic layer was washed with brine, dried (NA2SO4),filtered, and concentrated. Purification by flash chromatography (2: 1 EtOAc/hexanes) afforded the title compound as a dark yellow waxy solid (110 mg,48%).
【WO2003/99769】
三、
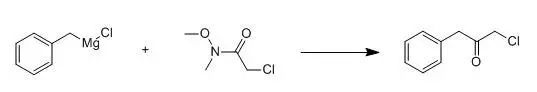
Benzylmagnesium chloride (1.0 M in diethyl ether, 50.0 mL) was treated with 2-chloro-N-methoxy-N-methylacetamide (5.16 g, 37.5 mmol) in THF (200 mL) at -78℃drop wise. The reaction was allowed towarm slowly to room temperature overnight and quenched with 1N hydrochloric acid. The layers wereseparated and the organic phase dried (Na2SO4), filtered,and the filtrate was concentrated under reduced pressure. The residue was purified by flash columnchromatography (silica gel, elution with hexanes) to provide the title compound.
四、
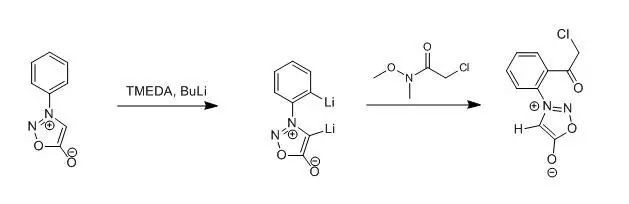
To a stirred solutionof 3-phenylsydnone (1) (0.25 g,1.54 mmol) in dry THF (100 ml) at -78℃under an atmosphere of dry nitrogengas was added N, N, N’, N’-tetramethylethylenediamine (0.29 ml, 1.93 mmol) then n-butyllithium (2.31 ml, 3.47 mmol, 15 Min pentane) dropwise. After 0.5 h, theappropriate 2-chloro-N-methoxy-N-methylacetamide (1.93 mmol) was added to thegolden yellow solution and, after a additional 1h, the mixture was quenchedwith aqueous hydrochloric acid (100ml, 10% v/v) then extracted withdichloromethane (3*100 ml). the combinedorganic layers were dried (MgSO4) and the solvent removed in vacuo to affordthe corresponding o-acylated sydnone 4 as an oil which was puriied by columchromatography to afford colorless crystals. Yield 86%.
【Turnbull,Kenneth; Sun, Congcong; Krein, Douglas M.; Tetrahedron Lett., EN; 39; 12; 1998;1509-1512】
五、通过Weinreb酰胺的还原得到醛

装备有温度计、搅拌子、滴液漏斗、空气冷凝回流管的5L圆底瓶中,通入氩气保护气后,加入LiAlH4(0.44 mol)以及无水乙醚(1.5L)。室温下搅拌一小时后,冷却到-45℃。将Boc-亮氨酸Weinreb酰胺(~100g, 約0.4mol)的无水乙醚溶液(300mL)、保持反应温度在-35℃以下缓慢滴加。加完后移去冷却槽,搅拌下让温度缓慢回复到5℃。再一次冷却降温到-35℃,将KHSO4(96.4g, 0.71mol)的水溶液(265mL)缓慢滴加,这时保持温度不要超过零度。去掉冷却槽,继续搅拌一小时。用硅藻土过滤反应液,用五百毫升乙醚洗净固体残渣。得到的有机溶液用1N盐酸(350mL)在5℃下洗净三次,再用饱和小苏打溶液(350mL)和饱和食盐水(350mL)先后分别洗净三次,用无水硫酸镁干燥除水。蒸干溶剂后,得到油状的Boc-L-Leucinal(69-70g,产率87-88%)。生成物放在冰柜(-17℃)保存。
【Goel, O. P.; Krolls, U.; Stier, M.; Kesten, S. Org. Synth. 1989, 67, 69.】
参考文献
一、化学空间:https://cn.chem-station.com/reactions/%e8%bf%98%e5%8e%9f%e5%8f%8d%e5%ba%94/2014/06/%e6%b8%a9%e5%8b%92%e4%bc%af%e9%85%ae%e5%90%88%e6%88%90-weinreb-ketone-synthesis.html
二、药明宝典
