
TMS2NCa类催化剂存在下,胺加成到烯烃上得到氢胺化产物的反应。ω-氨基烯烃进行分子内的此反应可以到吡咯烷类化物。除了Ca可以催化此反应,镧系金属也可以很好催化此反应,对于不对称催化,效果更好。
反应机理
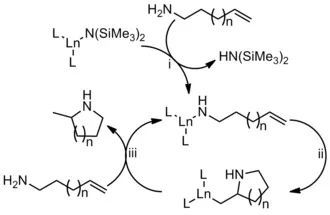
Pyrrolidine (4)(general procedure). Catalyst 2 (2–10 mol %) was added to 200–500 mg of aminoalkene 1 in 5 mL of PhH under Ar and the mixture was stirred until homogeneous. After quenching by exposure to air, rotary evaporation and bulb-to-bulb distillation gave 4, which was treated with HCl in
diethyl ether and analyzed by NMR.
【Hill MS, Barrett AGM, J Am Chem Soc., 2009, 131, 9670】

2-Benzylaminoethylbenzene (7).Styrene 5 (2.5 mmol) and benzylamine 6 (2.5 mmol) were treated under Ar with Ca{N(SiMe3)2}2 and the mixture was heated to 60 C for 48 h (faster reaction with Sr{N(SiMe3)2}2]). After exposure to air and dilution with Et2O, the suspension was filtered through Celite and evaporated to afford 2-benzyl-aminoethylbenzene 7 (oil, 92%), purified by chromatography or vacuum distillation.
【Hill MS, Barrett AGM, J Am Chem Soc., 2009, 131, 12906】
1 Marks TJ Acc Chem Res 2004 37 673
2 Hill MS J Am Chem Soc 2005 127 2042
3 Hill MS Organometal 2007 26 2953
4 Harder S Z Naturforsch 2008 63b 169
5 Hill MS, Barrett AGM J Am Chem Soc 2009 131 9670
6 Hill MS, Barrett AGM J Am Chem Soc 2009 131 12906
7 Harder S Chem Rev 2010 110 3852
编译自:Organic Syntheses Based On Name Reactions, 3RdEd, A. Hassner, Page 216-217.
